JXB Issue 75-03 – Editor’s choice
This article comments on:
Petutschnig EK, Pierdzig L, Mittendorf J, Niebisch JM, Lipka V. 2024. A novel fluorescent protein pair facilitates FLIM-FRET analysis of plant immune receptor interaction under native conditions. Journal of Experimental Botany 75, 746–759. https://doi.org/10.1093/jxb/erad418
Making the invisible visible
What goes on behind these walls? This fundamental question has fascinated generations of botanists ever since the first microscopic observation of plant cells in 1665, and it will surely entertain many more in the centuries to come. We have learned a great deal about the inner workings of plants since then, yet many of these discoveries were not made via direct imaging but rather indirect measurements followed by reasonable deduction. The colourful depictions of intracellular processes found in textbooks are, to a large extent, approximations of what can be concluded after extracting cellular content, shaking it in test tubes with some chemicals, running this cocktail through expensive machines, and looking at the numbers these instruments spit out for long enough. They are not real images of what is but thoughtful imaginations of what could be. For a long time, the closest scientists could get to a life-like depiction were cytological pictures taken from histological preparations of dead tissue after fixation and staining.
These were momentary snapshots frozen in time and space and revealed nothing about dynamic processes, leaving researchers in the dark about the internal activities that define life. However, a way to make the invisible visible had already been discovered in the mid-19th-century by the physicist George Gabriel Stokes in a solution of quinine. The transparent and colourless liquid lit up in a celestial blue when placed at the very edge of a spectrum of solar light dispersed by a prism. In awe of what he had witnessed, Stokes wrote: “It was certainly a curious sight to see the tube instantaneously light up when plunged into the invisible rays: it was literally darkness visible.
Altogether the phenomenon had something of an unearthly appearance.” It is this phenomenon that nightclubbers nowadays marvel at when enjoying their gin and tonic next to a black light, it is this phenomenon that elevated a Japanese marine biologist into the realm of Nobel Prize awardees, and it is this phenomenon that holds the power to shed light upon intracellular dynamics and revolutionised molecular biology. Stokes coined the light emission he observed ‘fluorescence’, and it occurs when certain atoms or molecules are excited by light of a particular wavelength and emit light of a longer wavelength after a very short time. Around a century after Stokes, Osama Shimomura collected nearly 10,000 specimens of the jellyfish Aequorea Victoria in Pacific waters to solve the mystery of the animal’s alluring glow, and successfully extracted a green fluorescent protein (GFP).
It would take another 30 years or so until GFP was sequenced and cloned, which enabled its expression in other organisms and made it a nowadays indispensable tools for non-invasive in vivo analysis of physiological activities on a sub-cellular or organismal level. Site-directed mutagenesis of the original GFP has yielded a vast array of fluorophores with different photophysical properties. Different proteins can thus be visualised simultaneous using spectrally separate fluorescent tags. Not only their localisation but also any potential interaction between proteins of interest is detectable in this way. For proteins to interact they must come into very close proximity of only few nanometres. When the distance between the attached fluorophores becomes small enough, the energy of a laser beam absorbed by one can be transferred to the other, which decreases the fluorescence lifetime and light emission yield of the donor fluorophore and increases the fluorescence of the acceptor. This so-called Förster resonance energy transfer (FRET) is recorded and analysed in fluorescence lifetime imaging microscopy (FLIM). However, using fluorescence analysis in plants is notoriously challenging, because of high internal autofluorescence from compounds such as chlorophyll or cell wall lignin. Signals from fluorescently labelled low abundant proteins are therefore oftentimes drowned out by inner “light pollution”.
Workarounds to increase the signal-to-noise ratio such as expressing proteins under stronger promoters can easily lead to artifacts and data misinterpretation. To overcome these limitations, Elena Kristin Petutschnig and colleagues have established a novel pair of fluorophores with superior properties, which they present in the Journal of Experimental Botany (Petutschnig et al., 2024). The fluorescent donor mCitrine and acceptor mScarlet-I (named after their respective yellow and red light emission) overlap in their light absorption and emission spectra, a prerequisite to study protein interaction using FRET/FLIM. They show excellent brightness and are thus ideal for proteins with low expression levels. The authors have validated the functionality of the fluorophores using two parts of a cell receptor complex that detects a fungal compound and triggers a plant defence response, and they revealed previously unknown details about the components’ interaction. The fluorophore pair has thus proven to shed new light on intracellular processes and the work holds much potential to further illuminate what goes on behind plant cell walls.
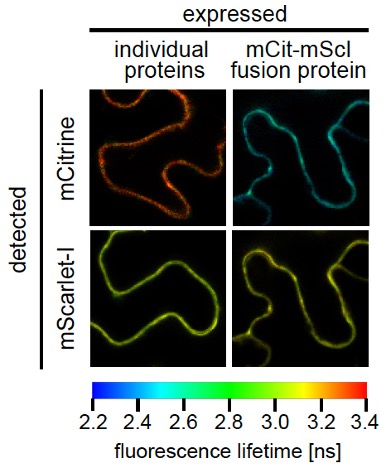
Petutschnig et al. (2024) present a new pair of fluorophores with excellent brightness that can be used for non-invasive in vivo analysis of protein interaction as illustrated in these epidermal cells of Arabidopsis plants. The left panel shows the fluorescence lifetime of the fluorophores when expressed individually. In the right panel, mCitrine and mScarlet-I were combined to form a fusion protein. The close proximity between the fluorophores enables energy transfer from the donor mCitrine to the acceptor mScarlet-I, which significantly decreases the donor lifetime.





