TPJ October 2023 Editor choice: "Breaking bad buttons: mescaline biosynthesis in peyote"
Research Highlight for:
Elucidation of the mescaline biosynthetic pathway in peyote (Lophophora williamsii)
https://doi.org/10.1111/tpj.16447
Breaking bad buttons: mescaline biosynthesis in peyote
The small, globular cactus peyote (Lophophora williamsii) is known for its ability to produce mescaline, a phenethylamine protoalkaloid (Figure 1). Consuming the mescaline-containing dried tops or “buttons” of peyote has a psychedelic effect that has been used in religious ceremonies by Indigenous communities for over 5800 years. Nowadays, psychedelic drugs are used for treatment of mental disorders such as depression, anxiety, or post-traumatic stress disorder. Mescaline is currently being tested in clinical trials, as its effects are longer lasting than those of other psychedelic drugs and might therefore provide greater therapeutic benefits.
Besides the phenethylamine alkaloids, there are tetrahydroisoquinoline (THIQ) alkaloids in peyote that are thought to act in the absorption, metabolism, and excretion of mescaline and thereby contribute to the psychedelic experience. Our current understanding of mescaline and THIQ biosynthesis is based on early radioisotope feeding experiments, which measured the incorporation of putative radiolabelled precursors into the biosynthetic products, but no mescaline or THIQ biosynthetic enzymes had been identified.
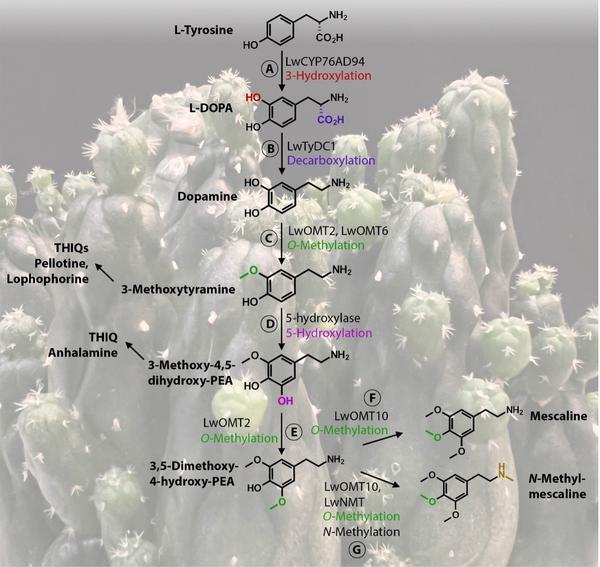
Figure 1: Mescaline biosynthesis pathway in peyote.
For mescaline and N-methylmescaline biosynthesis in peyote, L-tyrosine is 3-hydroxylated to L-DOPA (A), followed by decarboxylation to dopamine (B); dopamine undergoes 3-O-methylation to 3-methoxytyramine (C); then, an unknown 5-hydroxylase converts 3-methoxytyramine to 3- methoxy-4,5-dihydroxy-phenethylamine (PEA) (D); PEA is O-methylated to 3,5-dimethoxy-4-hydroxy-PEA, which is O-methylated yielding mescaline (E, F), or by N-methylation and 4-O-methylation into N-methylmescaline (G). The tetrahydroisoquinoline (THIQ) alkaloids are likely formed from the mescaline pathway intermediates 3-
Therefore, Watkins et al. set out to elucidate a near-complete biosynthetic pathway from L-tyrosine to mescaline in peyote, using both transcriptomics and a homology-guided gene discovery strategy. For that, the authors dissected epidermal and chlorenchyma tissue as one fraction, as well as the vasculature and cortex in another fraction, for transcriptome sequencing and assembly, and used untargeted metabolomics to measure the alkaloid accumulation. Both fractions contained low levels of mescaline and N-methylmescaline, but large quantities of the putative di-O-methylated intermediate, suggesting that all enzymes for the biosynthesis pathway were present, and enzymes that led to accumulation of the N-methylated derivative.
Conversion of L-tyrosine into mescaline requires hydroxylation of the benzene ring at the 3 and 5 positions before O-methylation. In sugar beet (Beta vulgaris), which like peyote belongs to the Caryophyllales, a member of the CYP76AD subfamily catalyses the 3-hydroxylation of L-tyrosine to L- DOPA. Therefore, the authors looked for homologs of the sugar beet enzyme based on amino acid identity, and found three members of the family in peyote. They transiently expressed the three candidates in yeast and found that LwCYP76AD94 catalysed the conversion of exogenous L-tyrosine to L-DOPA (Fig 1 A).
The conversion of L-tyrosine into mescaline requires aromatic amino acid decarboxylation (Figure 1B). The authors therefore searched for peyote homologs of the opium poppy tyrosine/DOPA decarboxylase. They found that LwTyDC1 was able to decarboxylate both L-tyrosine and L- DOPA. In a similar approach, the authors searched for homologs of the opium poppy 4’-O-methyltransferase 2 (OMT) and found that LwOMT10 catalysed the 4-O-methylation of dopamine, yielding 4-methoxytyramine (Fig 1C), as well as the 4-O-methylation of 3,5-dimethoxy-4-hydroxyphenethylamine (3,5- dimethoxy-4-hydroxy-PEA), yielding mescaline (Figure 1F), and N-methyl-3,5-dimethoxy-4-hydroxy-PEA, yielding N-methylmescaline (Figure 1G). LwOMT2 5-O-methylated the intermediate 3-methoxy-4,5- dihydroxyphenethylamine (3-methoxy-4,5-dihydroxy-PEA) (Figure 1E). Of the analysed enzymes, LwOMT3 had the largest substrate range. Because it had N-methyltransferase activity rather than O-methyltransferase activity, it was renamed LwNMT. It potentially produces N-methylated-mescaline (Fig 1G). N-Methylation of dopamine or 3-methoxytyramine could be the first step in the production of the N-methylated THIQ alkaloids.
The authors identified most mescaline biosynthetic enzymes and could thereby construct the biosynthesis pathway, although the enzyme that catalyses the 5-hydroxylation of the benzene ring remained elusive (Figure 1D). The strategy of a homology-guided gene discovery can limit the discovery of novel enzymes because it assumes amino acid homology on as a start point. Other techniques, such as co-expression analysis or gene clustering, might be useful to identify the hydroxylase enzyme.
Due to habitat loss and overharvesting, peyote is an endangered species. As an alternative to its extraction from peyote, mescaline can be synthesised chemically. The identification of the biosynthetic pathway will allow the development of synthetic biosystems for mescaline production on an industrial scale.





