TPJ Editor choice: April 2024: Detecting the ebb and flow of a phytohormone: a ratiometric biosensor to analyse gibberellin dynamics
Highlighted publication:
Ratiometric gibberellin biosensors for the analysis of signaling dynamics and metabolism in plant protoplasts
https://doi.org/10.1111/tpj.16725
Detecting the ebb and flow of a phytohormone: a ratiometric biosensor to analyse gibberellin dynamics
The plant hormone gibberellin (GA) serves as a pivotal regulator of plant development, controlling crucial processes such as seed germination, vegetative growth and the induction of flowering. The processes of GA biosynthesis, catabolism, conjugation and transport are finely tuned by intrinsic and external signals, resulting in highly dynamic GA patterns that control cell division and. Understanding these multi-faceted mechanisms requires rapid and reliable detection of GA accumulation with high spatiotemporal resolution. While traditional methods such as mass spectrometry are inherently destructive and provide limited resolution, there are now genetically-encoded fluorescent biosensors that either directly detect GA molecules or signalling processes downstream of GA perception in planta.
In the highlighted publication, Andres et al. expand the toolkit for GA detection by developing and testing ratiometric, degradation-based biosensors with improved sensitivity for high throughput experiments in transient expression systems. These sensors are based on components of the intrinsic GA perception machinery, the DELLA transcriptional regulators, which encompass GA-INSENSITIVE (GAI), REPRESSOR OF ga1-3 (RGA), RGA-LIKE 1 (RGL1), RGL2 and RGL3. In the absence of GA, DELLA proteins suppress transcriptional GA responses. When GA binds the GA receptor GA INSENSITIVE DWARF 1 (GID1), a complex forms between GID1, DELLAs and the F-box protein SLEEPY 1 (SLY1). SLY1 is part of an SKP1/CUL1/F-box (SCF) E3 ubiquitin ligase complex that catalyses the polyubiquitination of DELLA proteins, thereby triggering their proteasomal degradation and alleviating the repression of GA responses (Fig. 1a). For their biosensors, Andres et al. fused the DELLA sequences to a firefly luciferase (FF) reporter; a renilla luciferase (REN) was encoded in the same transcript, separated by a 2A ribosomal skipping sequence. This design enabled the observation of DELLA biosensor degradation via FF luminescence, with REN luminescence being used as a signal for normalisation (Fig. 1b).
These DELLA-based biosensors were tested in Arabidopsis wild-type protoplasts. All biosensors exhibited a reduced FF/REN luminescence ratio with increasing concentrations of bioactive GAs, while the luminescence ratio remained unchanged for negative controls. Sensitivity varied among sensors: RGA-FF exhibited remarkable sensitivity, showing a response even at very low concentrations of 10 pM GA4 or GA7, while RGL3 required 100 nM GA4 for a discernible reduction in luminescence ratio. Additionally, the degradation response was rapid, with RGA-FF displaying detectable degradation within 30 minutes of exposure to micromolar concentrations of GA4.
Once the authors were confident that their DELLA-FF biosensors could reliably report changes in GA concentration, they set out to tackle biological questions concerning GA metabolism and transport: treatment with the GA precursors GA9 and GA20 showed that GA9 triggered a reduction in FF/REN luminescence ratio of all DELLA-FF sensors at significantly lower concentrations than GA20, suggesting that GA9 is more readily converted into bioactive GA. Moreover, RGA-FF was used to assess the efficacy of GA2ox catabolic enzymes: overexpression of GA20ox1 or GA20ox2, but not of GA20ox8, resulted in reduced degradation of RGA-FF. Finally, RGA-FF confirmed the in-planta GA transport activity of NRT1/PTR FAMILY 3 (NPF3), a protein previously shown to transport GA in yeast and Xenopus oocytes. Co-expression of RGA-FF and NPF3 enhanced the drop in FF/REN luminescence ratio in the presence of GA3, but not GA4, suggesting that NPF3 exhibits selectivity for transporting GA3 over GA4.
In summary, Andres et al. demonstrated the suitability of their DELLA-FF biosensors for user-friendly quantification of GA dynamics with moderately high throughput, making them ideal tools for screening approaches. In addition, the exceptionally high sensitivity of RGA-FF surpasses that of existing sensors, making it an excellent tool for monitoring metabolic and signalling processes at low, physiologically relevant GA concentrations. The authors thus see their DELLA-FF biosensors complementing the suite of existing GA biosensors: FRET-based GA Perception Sensor 1 (GPS1) is primarily designed to quantify endogenous hormone levels in intact tissues (Rizza et al., 2017) while Hormone-Activated Cas9-based Repressors (HACRs) can be used to manipulate cellular processes in response to GA (Khakhar et al., 2018). Collectively, these tools will advance our understanding of the intricate mechanisms governing GA accumulation and signalling throughout plant development.
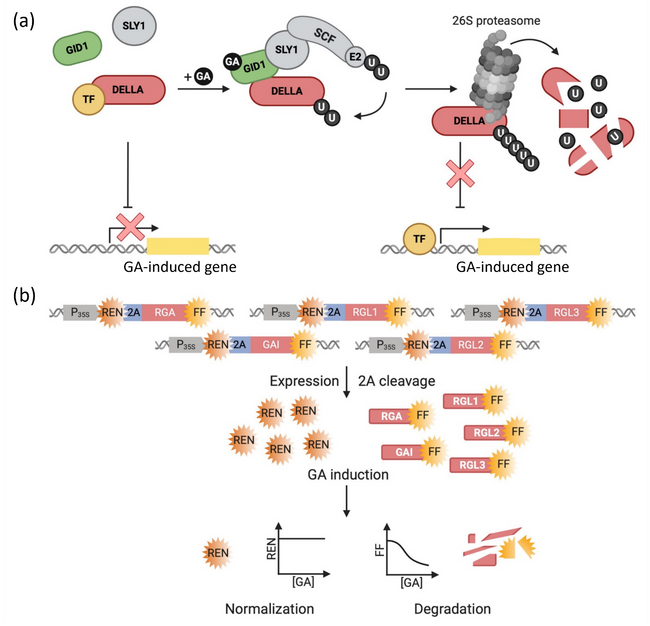
Figure 1. The mechanism of gibberellin (GA) perception in Arabidopsis thaliana and its exploitation for DELLA-based biosensor design.
- In the absence of GA, DELLA proteins interact with transcription factors (TFs) to suppress transcriptional GA responses. Upon GA binding, a complex forms between the GA receptor GID1, DELLA proteins and the F-box protein SLY1. SLY1 is part of an SKP1/CUL1/F-box (SCF) E3-ubiquitin ligase that catalyses the polyubiquitination of DELLAs, targeting them for degradation in the 26S proteasome and thereby alleviating the repression of GA responses.
- DELLA-FF biosensors contain one DELLA sequence as a sensory module fused to a firefly luciferase (FF) reporter. A renilla luciferase (REN) is encoded by the same transcript, separated by a 2A ribosomal skipping site, resulting in stoichiometric co-expression of DELLA-FF and REN. In the presence of GA, DELLA-FF is polyubiquitinated and consequently degraded, while REN levels remain constant, leading to a decrease in the FF/REN luminescence ratio.





