December 2025 TPJ Editor choice: New patterns in the wall: β-1,2-xylosyltransferases catalyse a novel, conserved xylan modification
Highlighted publication:
New patterns in the wall: β-1,2-xylosyltransferases catalyse a novel, conserved xylan modification
Plant cell walls provide essential structural support and protection. Although primarily composed of cellulose fibrils, they also contain other complex polysaccharides, such as pectin and hemicelluloses. Xylan, a hemicellulose composed of β-(1→4)-linked D-xylose units, is the second most abundant biopolymer on Earth after cellulose. Its backbone can carry a diverse range of side-chain substitutions that vary across species, tissues and developmental stages, shaping its conformation and interactions with other cell wall components and thereby cell wall properties.
Xylan substitution patterns are well described in secondary cell walls of flowering plants, but far less is known about xylan in primary cell walls, where it is present at much lower levels. Temple and colleagues set out to determine which modifications decorate xylan in primary cell walls of gymnosperms. To this end, they isolated alcohol-insoluble material from needles (containing both primary and secondary walls) and wood (rich in secondary walls) of several conifer species. Enzymatic fingerprinting followed by polyacrylamide carbohydrate gel electrophoresis showed distinct hydrolysis patterns of polysaccharides: digestion with a GH11 xylanase produced bands differing between needles and wood, particularly among high molecular weight, substituted oligosaccharides.
Using carefully designed combinations of glycosidase treatments, the authors further characterised two oligosaccharides, designated A and Y, that accumulated primarily in needles. Both were sensitive to digestion with α-glucuronidase GH115, indicating the presence of glucuronic acid (GlcA) or methylated GlcA (MeGlcA) sidechains (Fig. 1). Oligosaccharide A was also digested by arabinofuranosidase GH51, confirming that it carries L-α-arabinofuranose and likely corresponds to a previously known oligosaccharide. In contrast, oligosaccharide Y was resistant to GH51 but susceptible to GH62 (Fig. 1), which the authors unexpectedly found to have both arabinofuranosidase and xylosidase activity. MALDI-CID mass spectrometry and solution-state NMR analysis eventually revealed that Y contains a hexameric xylose backbone with a β-1,2-linked xylosyl residue at position −3 – a xylan substitution previously unknown in gymnosperms.
Which enzymes might generate this pattern? In vitro assays using heterologously-expressed xylan β-1,2-xylosyltransferases (XYXTs) from the conifer Pseudotsuga menziesii and Arabidopsis xylan as substrate showed that PmXYXT2 produced an oligosaccharide pattern resembling the conifer needle fingerprint. Consistent results were obtained in vivo: expressing PmXYXT2 in Arabidopsis produced the same diagnostic GH51-resistant, GH62-sensitive band. Further structural analysis with position-specific hydrolases and MALDI-CID mass spectrometry showed that PmXYXT2 installs a β-1,2-xylosyl residue positioned +3 relative to a MeGlcA substitution, generating an evenly spaced MeGlcA–xylose motif in primary cell wall xylans.
β-1,2-xylosylation appears to be more widespread than initially thought. Enzymatic fingerprinting of Arabidopsis tissues showed a GH51-resistant, GH62-sensitive oligosaccharide in leaves and especially in callus, both rich in primary cell walls. The authors identified three putative Arabidopsis homologues of PmXYXT2, designated AtXYXT1-3. AtXYXT1 produced the same GH62-sensitive patterns as PmXYXT2 when ectopically expressed in Arabidopsis. Enzymatic fingerprinting and MALDI-CID confirmed that AtXYXT1 installs a similar MeGlcA-dependent β-1,2-xylosylation pattern.
What role might β-1,2-xylosylation play in vivo? Analyses of the Arabidopsis xyxt mutants provided some initial clues. Levels of β-1,2-xylosylated oligosaccharides were reduced in single and double mutants and were completely absent in the xyxt1 xyxt2 xyxt3 triple mutant. As previously reported, xyxt1 mutants showed defects in mucilage adherence during seed hydration, but the triple mutant also displayed delayed senescence, suggesting a broader developmental role for this modification. The molecular function of β-1,2-xylosylation, however, remains unknown. Xylan interacts with pectin and possibly cellulose, with the latter interaction depending on evenly spaced xylan substitutions. Temple suggests that loss of β-1,2-xylosylation might disrupt xylan-pectin-cellulose interactions, altering cell wall architecture and remodelling.
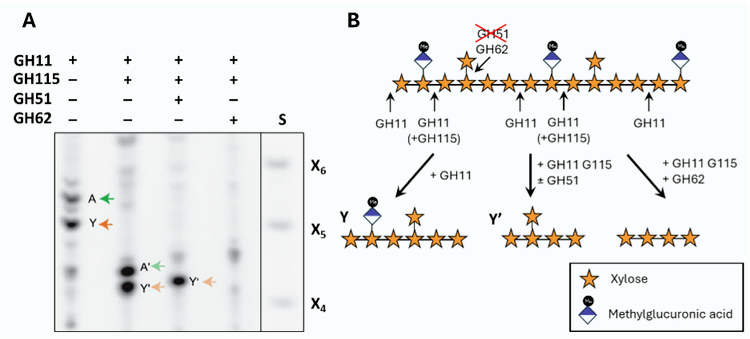
Figure 1. Identification of a new 1,2-xylosylated xylo-oligosaccharide in conifer needles.
- Alcohol-insoluble material from Picea abies digested with glycosyl hydrolases as indicated and separated by polyacrylamide carbohydrate gel electrophoresis (PACE). A and Y (and their digestion products A’ and Y’) indicate oligosaccharides preferentially detected in material with primary cell walls.
- Schematic representing selected hydrolysis reactions detected by PACE. Note that an additional position becomes accessible to G11 if MeGlcA is removed by GH115.



