June 2025 TPJ Editor choice: High temperature‐responsive DEAR4 condensation confers thermotolerance through recruiting TOPLESS in Arabidopsis nucleus
High temperature‐responsive DEAR4 condensation confers thermotolerance through recruiting TOPLESS in Arabidopsis nucleus
https://doi.org/10.1111/tpj.70172
How DEAR4 keeps plants cool under pressure
Liquid–liquid phase separation (LLPS) describes a process in which biomolecules spontaneously separate from a uniform solution into two liquid layers - a dense condensate phase and a dilute phase. These so-called biomolecular condensates are composed of concentrated proteins and nucleic acids and can be found in the nucleus, the cytoplasm and membranes. They play roles in many biological processes, including transcriptional regulation, RNA splicing, protein degradation and stress granule formation.
In plants, stress granule formation is a mechanism for temperature sensing: high temperature induces a condensation of thermosensors by LLPS into nuclear subdomains, where they interact with heat stress- responsive genes. Condensate formation by LLPS is also a way to integrate light signalling with a response to high temperature. Overexpression of the DEHYDRATION-RESPONSIVE ELEMENT BINDING (DREB) transcription factor, DREB AND EAR MOTIF PROTEIN 4 (DEAR4), causes hypocotyl elongation under both far- red/red and blue light. Since some DEARs are also responsive to temperature stress, Wang and colleagues analysed the response of DEAR4 overexpression to high temperature.
They found that DEAR4 overexpressors had a higher survival rate under elevated heat stress temperature. Interestingly, under heat stress, the proteins formed speckle-like condensations in the nuclei of Arabidopsis roots, which was reversible upon a return to normal temperature. To analyse the nature of the condensates, the authors used fluorescence recovery after photobleaching (FRAP). Unlike solid aggregates, the condensates formed by LLPS exhibit liquid-like behaviours such as fluidity, fusion, and dripping. In FRAP experiments, fluorescently-labelled molecules within a localized region of the condensate are photobleached. The subsequent recovery of fluorescence signal is monitored as unbleached molecules diffuse back into the bleached area. Rapid fluorescence recovery, which the authors observed for DEAR4-GFP, indicates liquid-like properties, as it demonstrates fast molecular redistribution and dynamic exchange within the condensate.
Luciferase assays and overexpression of truncated protein versions lacking the ERF-associated amphiphilic repression (EAR) domain showed that the EAR motif is crucial for transcriptional repression of target genes and DEAR4-mediated thermotolerance. As transcriptional targets the authors identified GA-STIMULATED ARABIDOPSIS 5 (GASA5), a negative regulator of some heat shock proteins under heat stress. TOPLESS and TOPLESS-RELATED PROTEIN 1 (TPR1) were identified as protein interactors. As expected, the interaction was facilitated by the EAR motif, and the DEAR4-TPL complex formed speckle-like structures, with more speckles forming under higher temperatures.
The C-terminus of DEAR4 contains a long intrinsically disordered region (IDR), which is often associated with LLPS. The EAR motif within the IDR region had the highest Predictor of Natural Disordered Regions score, therefore the authors tested the relevance of this motif for the condensation. DEAR4-GFP without the EAR motif in the IDR region did not form condensates under high temperature, suggesting that this motif is important for LLPS-mediated condensation.
TPL/TPR corepressors interact with histone deacetylases to regulate chromatin. The H3K9ac histone acetylation level of GASA5 was reduced under heat stress in both wild type and DEAR4 overexpressors, and the heat stress- triggered repression of GASA5 expression was attenuated in tpl mutants, suggesting that the inhibition of GASA5 expression could be caused by histone deacetylation.
In this study, the authors showed that DEAR4 forms reversible, LLPS-dependent speckles under high temperature. In these speckles, DEAR4 interacts with TPL corepressors to repress the negative thermotolerance regulator GASA5 through chromatin modification. This demonstrates another way how LLPS-mediated speckle formation could be used to integrate heat stress into transcriptional pathways.
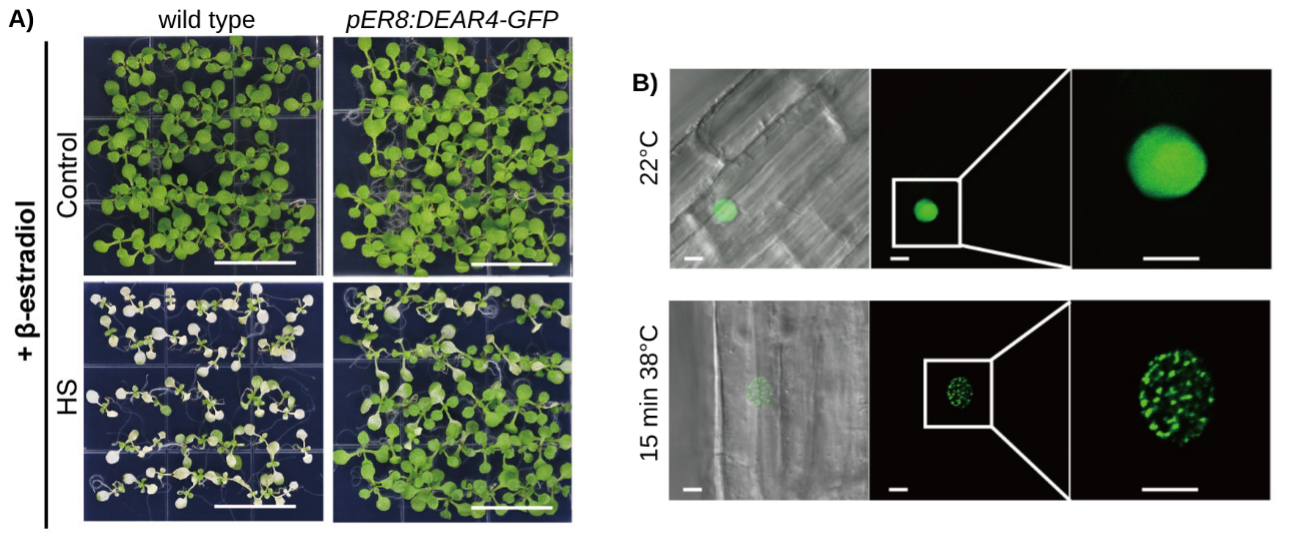
Figure 1: DEAR4 confers thermotolerance in Arabidopsis.
A) β-estradiol-induced overexpression of DREA4 increases the seedling survival rate under heat stress (HS).
B) High temperature induces the formation of DEAR4-GFP nuclear condensates.
Modified from (Wang et al., 2025).





